
įrom the 16th century, researchers including Jan Baptist van Helmont, Robert Boyle, and Isaac Newton tried to establish theories of experimentally observed chemical transformations. Further optimization of sulfuric acid technology resulted in the contact process in the 1880s, and the Haber process was developed in 1909–1910 for ammonia synthesis.
With the development of the lead chamber process in 1746 and the Leblanc process, allowing large-scale production of sulfuric acid and sodium carbonate, respectively, chemical reactions became implemented into the industry. In the 17th century, Johann Rudolph Glauber produced hydrochloric acid and sodium sulfate by reacting sulfuric acid and sodium chloride. The production of mineral acids involved the heating of sulfate and nitrate minerals such as copper sulfate, alum and saltpeter. 850–950) attributed to Jābir ibn Ḥayyān, or the production of mineral acids such as sulfuric and nitric acids by later alchemists, starting from c. Examples include the synthesis of ammonium chloride from organic substances as described in the works (c. The artificial production of chemical substances already was a central goal for medieval alchemists. They attempted, in particular, to convert lead into gold, for which purpose they used reactions of lead and lead-copper alloys with sulfur. In the Middle Ages, chemical transformations were studied by alchemists. Initial theories of transformation of materials were developed by Greek philosophers, such as the Four-Element Theory of Empedocles stating that any substance is composed of the four basic elements – fire, water, air and earth.

History Antoine Lavoisier developed the theory of combustion as a chemical reaction with oxygen.Ĭhemical reactions such as combustion in fire, fermentation and the reduction of ores to metals were known since antiquity. The general concept of a chemical reaction has been extended to reactions between entities smaller than atoms, including nuclear reactions, radioactive decays and reactions between elementary particles, as described by quantum field theory. Enzymes increase the rates of biochemical reactions, so that metabolic syntheses and decompositions impossible under ordinary conditions can occur at the temperature and concentrations present within a cell. These reactions are often catalyzed by protein enzymes.
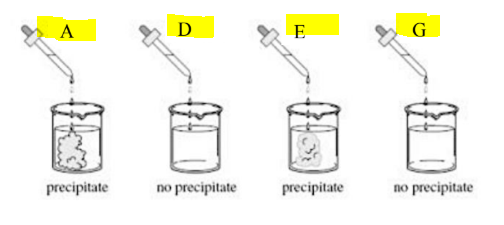
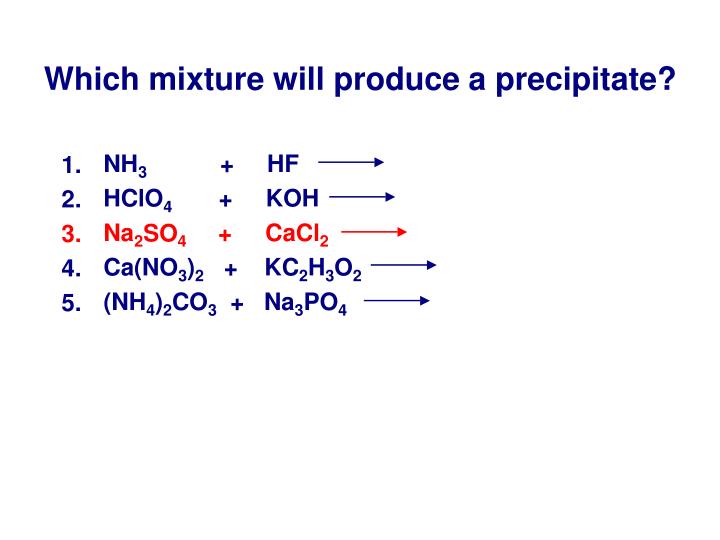
Which combination will produce a precipitate series#
In biochemistry, a consecutive series of chemical reactions (where the product of one reaction is the reactant of the next reaction) form metabolic pathways. Most simple redox reactions may be classified as a combination, decomposition, or single displacement reaction.ĭifferent chemical reactions are used during chemical synthesis in order to obtain the desired product. Typically, reaction rates increase with increasing temperature because there is more thermal energy available to reach the activation energy necessary for breaking bonds between atoms.Ī reaction may be classified as redox in which oxidation and reduction occur or non-redox in which there is no oxidation and reduction occurring. Chemical reactions are described with chemical equations, which symbolically present the starting materials, end products, and sometimes intermediate products and reaction conditions.Ĭhemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course of action is part of the reaction mechanism. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation.
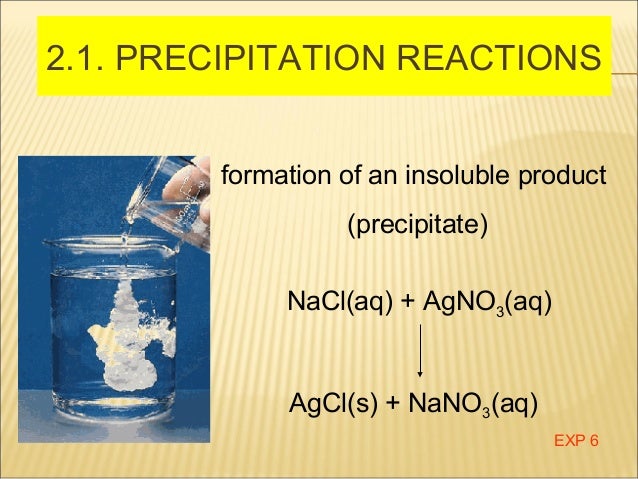
The sparks flying outwards are globules of molten iron trailing smoke in their wake.Ī chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. ( April 2023) ( Learn how and when to remove this template message)Ī thermite reaction using iron(III) oxide. Please help to improve this article by introducing more precise citations. This article includes a list of general references, but it lacks sufficient corresponding inline citations.


 0 kommentar(er)
0 kommentar(er)
